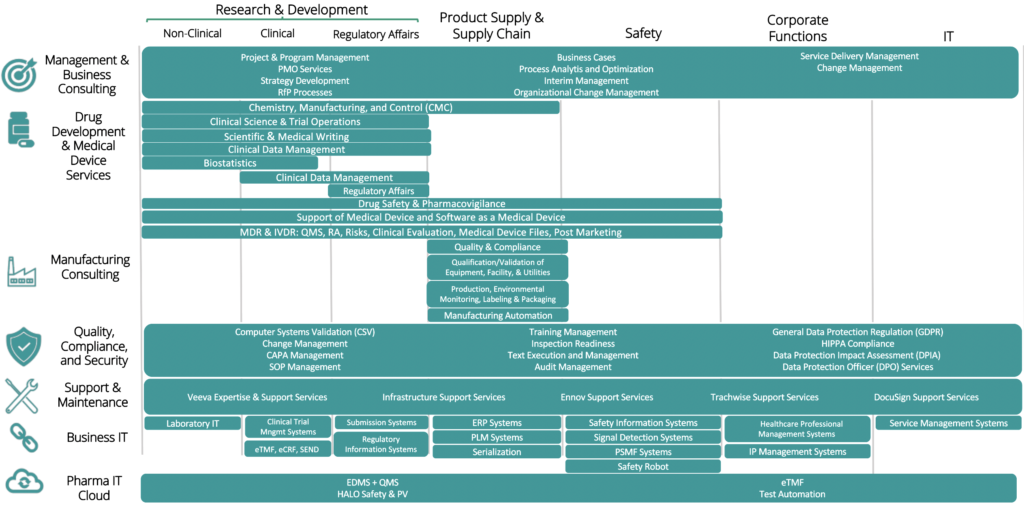
Pharma IT is a one-stop consultancy shop providing services exclusively for the pharma, biotech, and medical device industries
Our experienced consultants have extensive knowledge from the industry and they all understand the quality and compliance requirements that are the underlying foundation for work within pharma, biotech and medical device industries. Our young talent offers cost effective alternative either individually or supporting our experienced consultants.