When building, maintaining, or developing a QMS everybody is aware, that there need to be procedures, policies, and work instructions in place. But not that many thinks of them as them as processes.
That is too bad, because that is what they really are. And there is time to spare to get them front and center in your perception. And in your documentation.
When you have your processes in place – and here we are talking about having them:
- Identified
- Mapped
- Documented
- Aligned (internal and with i.e. external requirements)
- Risk assessed
- Updated with roles and responsibilities
– then you have your processes in place.
Processes visualize what we do, who is doing it, when it is being done and also; you can benefit largely if you add in the risk, that the process host, to ensure the overview of the various risk levels in your organization. Perhaps the risk is related to the business, the data or the sensitivity of the manufacturing flow – or something unique for your company.
At Pharma IT we can help you get your processes in place – we are only a click or a call away. So, go on – reach out and let us help you gain transparency in your Quality processes and make them visual management, to ease your busy schedule.
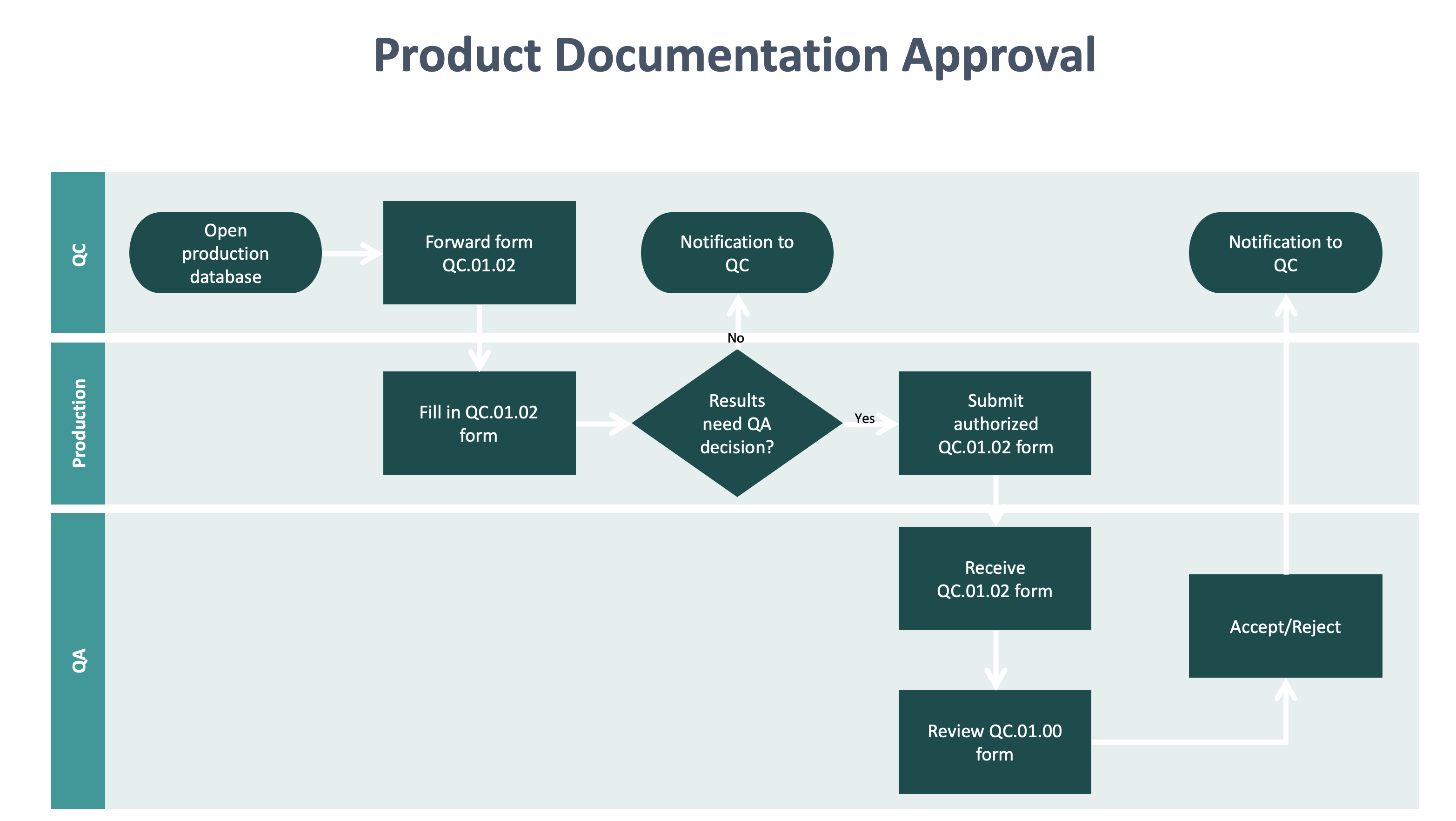
Process Drawing below:
Read more about our Medical Device team and services here