Share on email
Share on linkedin
Share on facebook
Share on whatsapp
Share on twitter
By Daniel Isaac Kemajou Njamen
September 26, 2024
By Daniel Isaac Kemajou Njamen
September 27, 2024
With the ongoing migration from the eXtended EudraVigilance Medicinal Product Dictionary (xEVMPD) to the Product Management Service (PMS), companies face the added challenge of ensuring seamless data integration and synchronization. This transition heightens the need to align internal systems with public health platforms to avoid regulatory pitfalls and preserve public trust.
In this Insight, we delve deeper into the challenges of meeting these regulatory demands and present an automated AI solution to solve them. Pharma IT’s innovative IDMP Gap Analysis Tool offers a powerful, interactive solution for identifying and closing compliance gaps in their product data.
The IDMP standard is a global framework, governed by the International Organization for Standardization (ISO) which aims to improve the transparency, accuracy, and safety of medicinal product data across the entire product lifecycle.
For pharmaceutical companies, the transition from xEVMPD to PMS introduces several new layers of complexity to IDMP adherence, especially in managing data from multiple sources. Key data points such as drug traceability, pharmacovigilance information, and lifecycle data must be consistent between internal systems (e.g. RIM, Veeva, and Regulatory Submissions systems) and public health platforms (e.g. databases managed by the European Medicines Agency (EMA) such as EMA’s PLM portal and EMA SPOR management services.
Inaccurate or outdated information on public health pages like those of the FDA, EMA, or ANSM can also erode public trust by undermining the credibility of a company and its products.
However, the manual effort required to keep public health information synchronized with internal systems is a significant concern. This manual process is not only time-consuming but also prone to human error, leading to further potential data mismatches.
To overcome these challenges, Pharma IT has developed an automated AI-powered IDMP Gap Analysis Tool, which provides an interactive approach to identifying and addressing compliance gaps:
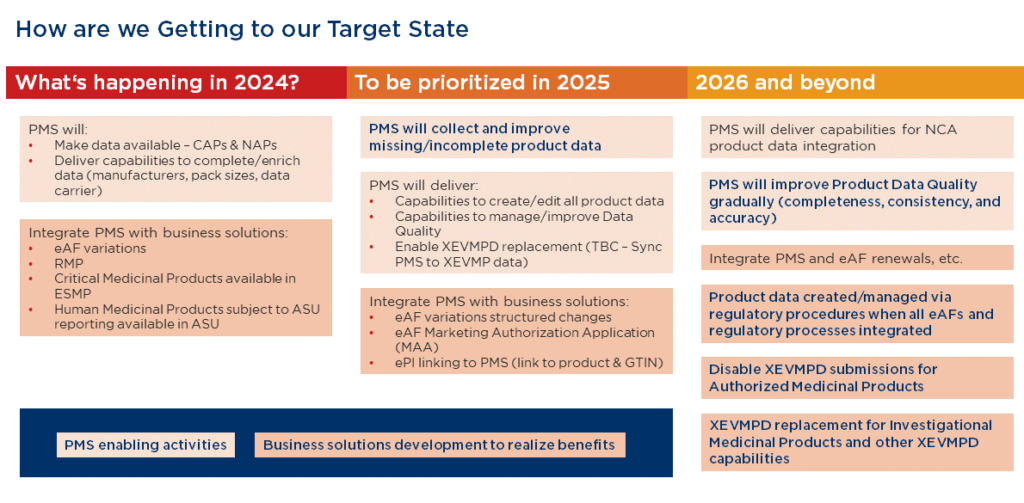
Figure 1: The EMA’s Roadmap for the transition to PMS, including the mighration of SIAMED and XEVMPD data. Source: European Medicines Agency
The transition from xEVMPD to PMS represents a major regulatory challenge for the pharmaceutical industry, but it also presents an opportunity to enhance data accuracy, operational efficiency, and public trust.
With this powerful tool, pharmaceutical companies can navigate the complexities of PMS migration with confidence, ensuring compliance, trust, and long-term success in an increasingly regulated market.
Pharma IT’s IDMP Gap Analysis Tool uses real-time, interactive insights to empower your compliance team to pinpoint and close gaps in product data.
Contact us today to schedule a demo to see firsthand how our tool can help you stay ahead of regulatory changes, reduce risk, and optimize your compliance strategy.


Daniel Isaac Kemajou Njamen is a Consultant at Pharma IT and a passion-driven software developer with a master’s degree in Mechatronics Engineering, where he profiled in Software Development. Daniel has performed the role of Software Developer within different industries, including the Medical Device Industry, where he developed code for Hearing Instrument features implementation and defect mitigation. He is proficient in C, C++, and Python programming languages and has hands-on experience with Agile Processes.