Scientific & Medical Writing
Proven excellence across clinical development and regulatory medical writing
Scientific & Medical Writing
Proven excellence across clinical development and regulatory medical writing
We provide expert medical writing services within all key therapeutic areas
Our highly qualified professionals possess consummate medical writing expertise backed by years of experience in preparing clinical, regulatory, pharmacovigilance, and medical marketing documents for the world’s top pharmaceutical companies.



We possess the technical know-how necessary to get your documents approval-ready
Our consultants are experienced in all forms of medical writing: From well-written protocols and comprehensive clinical study reports, to pertinent scientific manuscripts, investigator’s brochures, pharmacovigilance documents, and more.
Pharma IT's medical writing team consists of highly qualified professionals with clinical backgrounds.
“Our team has expertise in communicating to a wide range of audiences, as well as proven expertise in the biopharmaceutical sector across therapeutic areas and clinical development stages. This enables us to deliver complete, end-to-end medical writing services with impeccable attention to detail.”
Director of Clinical Science & Trial Operations
We provide expert medical writing services within all key therapeutic areas
Our highly qualified professionals possess consummate medical writing expertise backed by years of experience in preparing clinical, regulatory, pharmacovigilance, and medical marketing documents for the world’s top pharmaceutical companies.



We possess the technical know-how necessary to get your documents approval-ready
Our consultants are experienced in all forms of medical writing: From well-written protocols and comprehensive clinical study reports, to pertinent scientific manuscripts, investigator’s brochures, pharmacovigilance documents, and more.
Pharma IT's medical writing team consists of highly qualified professionals with clinical backgrounds.
“Our team has expertise in communicating to a wide range of audiences, as well as proven expertise in the biopharmaceutical sector across therapeutic areas and clinical development stages. This enables us to deliver complete, end-to-end medical writing services with impeccable attention to detail.”
Birgitte Sloth, MSc, PhD, PdDip
Director of Clinical Science & Trial Operations
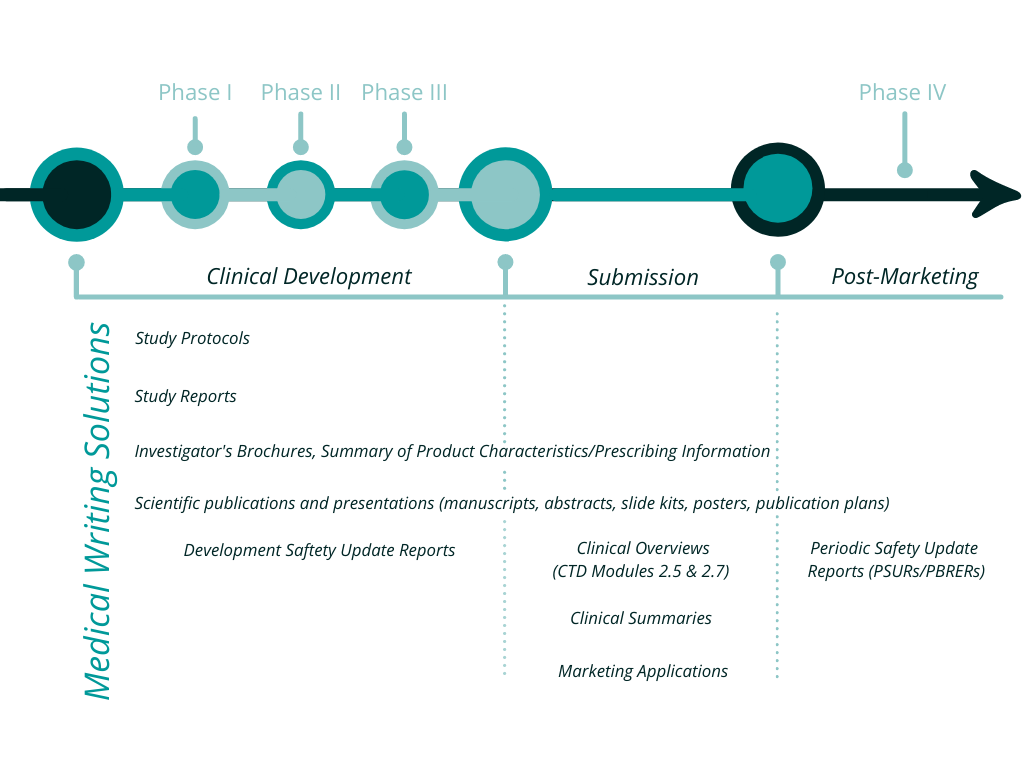
Full list of our Scientific & Medical Writing services:
- Investigator’s brochure (and updates)
- Clinical trial protocol (and amendments)
- Informed consent document
- Clinical trial report
- Clinical trial summary
- Post-marketing study reports/Health technology assessment reports
- Common technical document modules 2.5 and 2.7
- Summary of product characteristics/Prescribing information (and updates)
- Periodic benefit-risk evaluation reports
- Assistance with responses to regulatory authorities
- Scientific manuscripts for peer-reviewed journal publications
- Scientific abstracts, posters, and oral presentations (for scientific congresses)
- Systematic literature reviews
- Support for publication plans
- Disease and drug-related educational and promotional literature