Drug Safety & Pharmacovigilance
Full stack safety and pharmacovigilance services tailored to your needs
Drug Safety & Pharmacovigilance
Full stack safety and pharmacovigilance services tailored to your needs
We provide exceptional services to meet all of your drug safety and pharmacovigilance needs
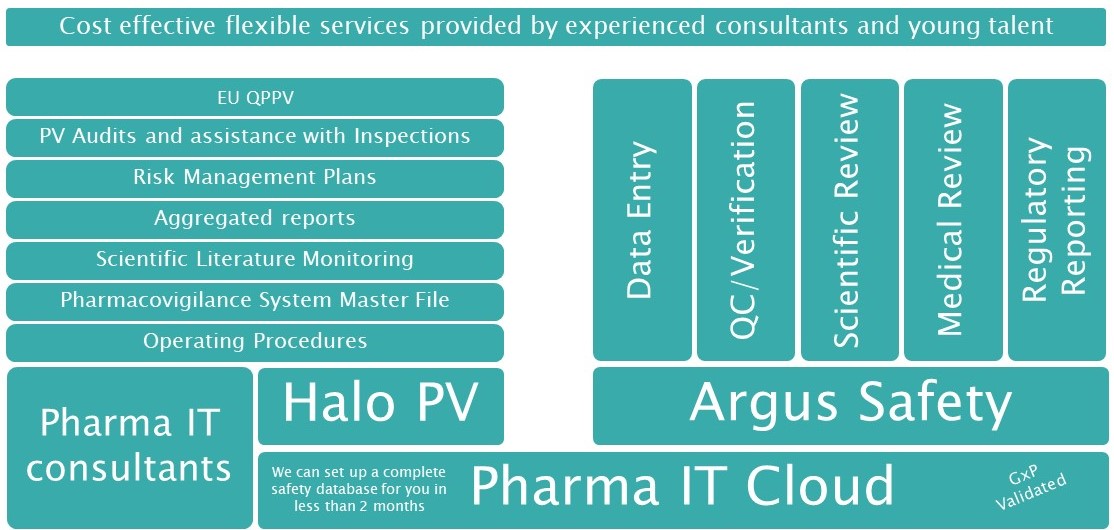
Our expert consultants can provide full stack drug safety and pharmacovigilance services tailored to your needs. Whether you need urgent help filling a temporary vacancy (maternity cover, illness, etc.) or are looking for a more permanent outsourcing solution. Our services can also be provided on top of Argus Safety and Halo PV, if needed.


Learn more about our offerings below
Our team is trained in data entry in the major used safety databases such as Argus and ARISg, and our consultants can handle every step from book-in, through data entry, quality control, safety review, medical review and distribution/reporting to authorities and partners. Pharma IT can also provide a safety database via the Pharma IT Cloud.
Our consultants can act as EU-QPPV/deputy QPPV for your company and offer assistance in setting up the necessary procedures to support this role. Before commencing the role as EU-QPPV we will perform a PV audit, to ensure that any discrepancies from an inspection-ready state are found and handled before the QPPV-services can begin.
If your local safety representative is absent, or you have a particularly busy period, we can help. Our consultants are all experienced with the pharmacovigilance legislation, and with safety training of sales staff, writing local SOPs, doing reconciliations and other tasks normally handled by your local safety representative. We can provide both short and longer term assistance.
If you are notified that you will be inspected by any regulatory authority, Pharma IT is ready to assist you with ensuring your process is inspection-ready. We also perform PV audits as an external partner working together with your QA-team or fully independent, depending on your set-up. Please note, that regulatory authorities expect that you perform regular audits of your PV system, and that these audits are conducted by external experts.
Since the release of the EU GVP Module V in March 2017, pharmaceutical companies have had to adapt to a new standard of reporting. Our consultants are experienced in the old and the new format and can help you transfer into the new format. Additionally, we are ready to help you with the content as well due to our experience across many different therapeutic areas. Our team is very experienced in writing other aggregated reports such as PSUR, DSUR, ACO and performing the benefit/risk assessments necessary for concluding on the safety of your product.
Although the European Medicines Agency (EMA) has started screening worldwide literature for some substances, pharmaceutical companies are still expected to monitor the scientific literature. Pharma IT can assist you with screenings of the literature, both locally and globally.
Our consultants are very experienced in maintaining and building the PSMF for small to large size pharmaceutical companies. The demands of the PSMF often need to be reviewed and complied with before the first marketing authorization is granted in the EU. To this end, please contact us, if you need strategic advice on how to set up your system most efficiently and compliantly. We can also assist you with an electronic solution to tailor and implement to maintain the PSMF via the Pharma IT Cloud.
Pharma IT services are delivered by experienced scientists and bright young talent depending on your needs
Our collective experience uniquely positions us to contribute specialist perspectives, experience, and efficiency. We tailor our services to each customer so you always get the most cost-effective solution.
















We can also optimize and support your daily safety processes with the Pharma IT Cloud
Based on our extensive knowledge about safety and pharmacovigilance processes Pharma IT can optimize and support your daily processes via IT. This includes offering a full safety database solution for streamlined and cost-efficient safety handling.
We provide exceptional services to meet all of your drug safety and pharmacovigilance needs
Our expert consultants can provide full stack drug safety and pharmacovigilance services tailored to your needs. Whether you need urgent help filling a temporary vacancy (maternity cover, illness, etc.) or are looking for a more permanent outsourcing solution. Our services can also be provided on top of Argus Safety and Halo PV, if needed.


Learn more about our offerings below
Our team is trained in data entry in the major used safety databases such as Argus and ARISg, and our consultants can handle every step from book-in, through data entry, quality control, safety review, medical review and distribution/reporting to authorities and partners. Pharma IT can also provide a safety database via the Pharma IT Cloud.
Our consultants can act as EU-QPPV/deputy QPPV for your company and offer assistance in setting up the necessary procedures to support this role. Before commencing the role as EU-QPPV we will perform a PV audit, to ensure that any discrepancies from an inspection-ready state are found and handled before the QPPV-services can begin.
If your local safety representative is absent, or you have a particularly busy period, we can help. Our consultants are all experienced with the pharmacovigilance legislation, and with safety training of sales staff, writing local SOPs, doing reconciliations and other tasks normally handled by your local safety representative. We can provide both short and longer term assistance.
If you are notified that you will be inspected by any regulatory authority, Pharma IT is ready to assist you with ensuring your process is inspection-ready. We also perform PV audits as an external partner working together with your QA-team or fully independent, depending on your set-up. Please note, that regulatory authorities expect that you perform regular audits of your PV system, and that these audits are conducted by external experts.
Since the release of the EU GVP Module V in March 2017, pharmaceutical companies have had to adapt to a new standard of reporting. Our consultants are experienced in the old and the new format and can help you transfer into the new format. Additionally, we are ready to help you with the content as well due to our experience across many different therapeutic areas. Our team is very experienced in writing other aggregated reports such as PSUR, DSUR, ACO and performing the benefit/risk assessments necessary for concluding on the safety of your product.
Although the European Medicines Agency (EMA) has started screening worldwide literature for some substances, pharmaceutical companies are still expected to monitor the scientific literature. Pharma IT can assist you with screenings of the literature, both locally and globally.
Our consultants are very experienced in maintaining and building the PSMF for small to large size pharmaceutical companies. The demands of the PSMF often need to be reviewed and complied with before the first marketing authorization is granted in the EU. To this end, please contact us, if you need strategic advice on how to set up your system most efficiently and compliantly. We can also assist you with an electronic solution to tailor and implement to maintain the PSMF via the Pharma IT Cloud.
Our services are delivered by experienced scientists and bright young talent
Our collective experience uniquely positions us to contribute specialist perspectives, experience, and efficiency. We tailor our services to each customer so you always get the most cost-effective solution.
















Do you need assistance with Drug Safety & Pharmacovigilance?
Reach out to discuss how our team can best support you


We can also optimize and support your daily safety processes with the Pharma IT Cloud
Based on our extensive knowledge about safety and pharmacovigilance processes Pharma IT can optimize and support your daily processes via IT. This includes offering a full safety database solution for streamlined and cost-efficient safety handling.