The EMA SPOR (ISO IDMP) Task Force is continuously working on implementing the ISO IDMP standard.
According to the EMA Substance and products data management services page the following apply:
- Product and substance data preparatory phase is ongoing and started after the launch of the RMS and OMS in 2017
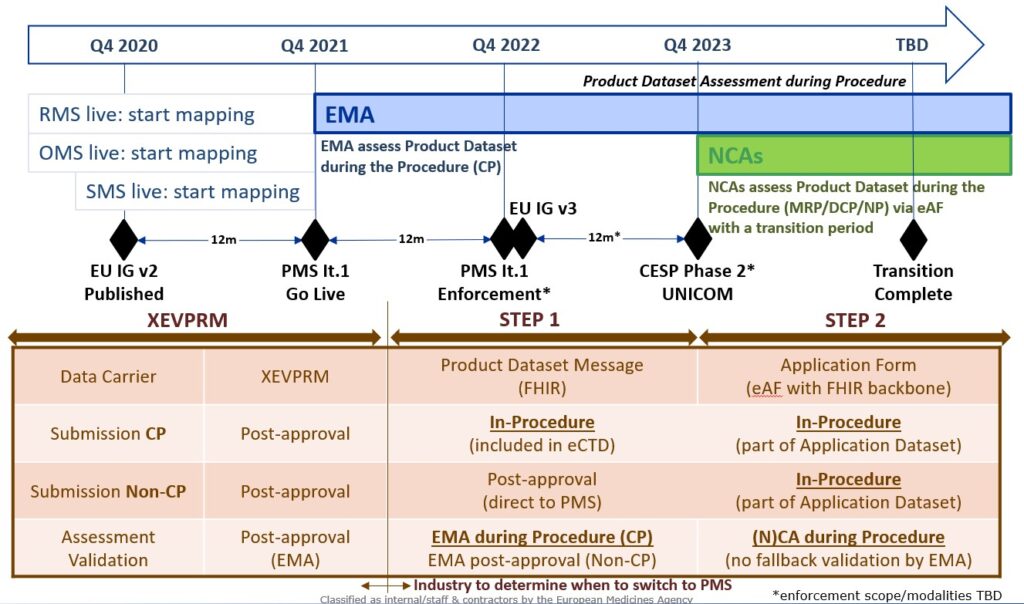
- Next phase is the “Product and substance data transition phase” which is planned to start no sooner than twelve months after EMA publishes EU IG v2 (Q4 2021)
- The final phase “Product and substance data submission phase” which will make submissions of IDMP product and substance data mandatory is planned to start no less than twelve months after the start of the transition phase (Q4 2022)
So if EMA publish the EU Implementation Guide version 2 by Q4 2020 IDMP submission of product and substance data might become mandatory by Q4 2022 for Centralised Procedures (CP) – MRP/DCP and National Procedures will not be applicable before 2023.
Planning input from the SPOR Task Force/IRISS Forum from april 2020 can seen below.
If you need support don’t hesitate to contact us.