
Share on email
Share on linkedin
Share on facebook
Share on whatsapp
Share on twitter
By Martin Rother Breyen
January 23, 2023
By Martin Rother Breyen
January 23, 2024
It is hard to ignore the rise in hype surrounding artificial intelligence (AI) applications across industries. The pharmaceutical industry is generally slower to adopt these technologies due to its highly regulated nature. This Insight is part of our AI in Pharma Series, where we share use cases for AI in a highly regulated reality.
Commercialization, marketing, and patient communication occur during the final stages of the drug development life cycle. These processes happen post-production, when a product has been registered and approved to be sold in a particular market.
Some examples of commercial activities include:
Promotional content refers to material or communications created to market or promote pharmaceutical products. This content informs healthcare professionals, patients, or the general public about specific medications, their benefits, and their intended uses. Therefore, it is also subject to strict regulations to ensure accuracy, transparency, and compliance with relevant laws. Promotional content may include advertisements, brochures, websites, or other materials.
Here are two use cases that describe how AI could be used to help meet these goals.
Pharmaceutical companies often hire external creative agencies to help them create content. This makes content ideation and creation both time and resource intensive. To lessen dependence on expensive external agencies, internal commercial teams could leverage AI to create components of promotional materials themselves.
There are hundreds of AI tools on the market (see Figure 1). For photos, graphics, and logo creation, companies can take advantage of AI tools like Dalle-3 or Midjourney. Teams needing animations, stock photos, covers, and straps can utilize Midjourney and Stable Diffusion. Lovo can be used for vocal content, sound effects, and music creation. Finally, SlidesAI and secured GBT/LLM models can help you generate text, review text and support templates. A secure closed language model source is needed for this operation.
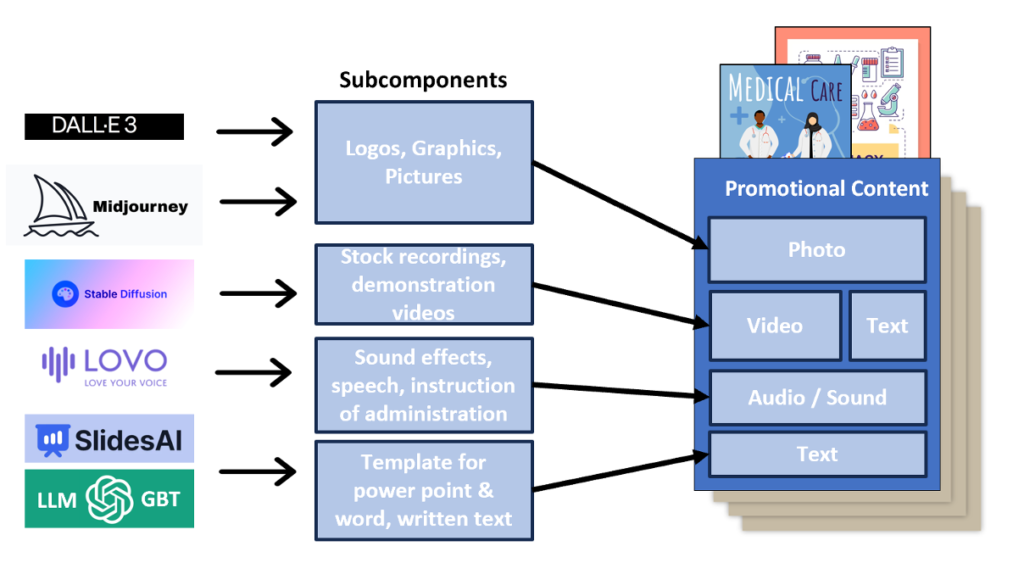
Figure 1. Examples of AI platforms available to create components of promotional materials.
This includes photos, videos, audio, text, and templates.
To meet regulatory requirements, promotional content must withstand a rigorous MLR (Medical, Legal, and Regulatory) review process before it is pushed out to market channels. AI can be utilized during this process to increase efficiency. For example, AI tools could pre-screen promotional content before teams begin their MLR reviews. This initial review could push clearly non-compliant content into the “not approved” portion of the workflow. In this case, the company’s SMEs would not waste time on an MLR review of content that is clearly not compliant.
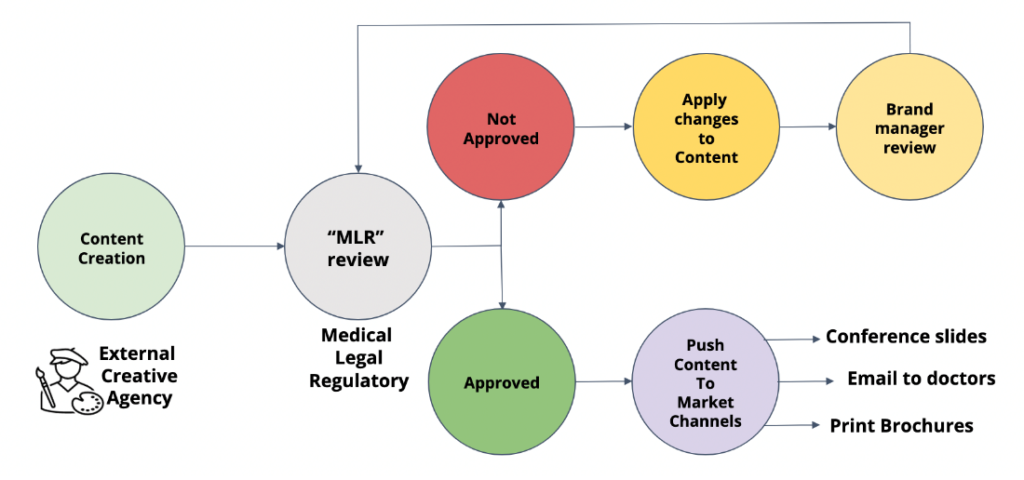
Figure 2. A simplified illustration of a basic content creation and approval process flow for promotional content used by pharmaceutical companies.
We’re helping customers identify and execute AI use cases across the drug development life cycle. We work alongside their teams to pilot these use cases and evaluate their benefits and pitfalls. This process helps teams gain AI experience and enables managers to decide which AI use cases could be beneficial at scale.
You can contact our team directly by clicking the button below. For commercial applications, you can reach out to Martin Rother Breyen directly at MaRo@pharmait.dk or +45 7196 2131.
Martin Rother Breyen is the Director of Commercial & Medical Consulting at Pharma IT. He holds more than 10 years’ experience in the pharmaceutical industry working with IT implementation, Omnichannel, Digital Asset Management, Veeva & Salesforce implementations, Veeva architecture, Veeva technical integrations and commercial / medical processes.