
Share on email
Share on linkedin
Share on facebook
Share on twitter
Share on skype
By Jakob Juul Rasmussen
December 16, 2021
By Jakob Juul Rasmussen
December 16, 2021
Many small-scale companies are currently using non-expandable platforms. Others use legacy EDMS+QMS solutions, which can be expensive up front and costly in the long term. With this in mind, we developed our Pharma IT Cloud EDMS+QMS solution in partnership with Ennov.
Our solution provided Ascelia Pharma with a secure, and fully GxP-compliant EDMS+QMS solution and fills this unique gap in the market.
Ascelia Pharma is a Swedish biotech company headquartered in Malmö. The company’s focus is on orphan oncology treatments. Ascelia Pharma prides itself on developing and commercializing novel drugs that address unmet medical needs and have a clear development and market pathway. The company has two drug candidates – Orviglance® (former working name Mangoral) and Oncoral – and is experiencing growth as these candidates enter advanced clinical development.
Regulatory bodies place stringent requirements on pharma companies that must be met, regardless of staff size
Ascelia Pharma needed a safe and secure place to store regulatory documentation and submission documentation that was streamlined and convenient to ease the burden of meeting these regulatory requirements
This would be the first large scale IT implementation undertaken at the firm, a challenge given its small team of approximately 20 staff
We partnered with Ascelia Pharma to implement our Pharma IT Cloud EDMS+QMS solution – a complete GxP-validated Document, Quality, and Training Management system based on Ennov software, and pre-configured according to industry best practices.
Our solution is hosted by Ennov. This platform provided a solid foundation to build upon due to its intuitive implementation and configuration, flexibility, secure and stable technology, and single repository solution.
While the Pharma IT Cloud can be configured for businesses of all sizes, it was designed specifically with small- and medium-sized businesses, like Ascelia Pharma, in mind. As a cost-effective alternative to legacy solutions, the Pharma IT Cloud EDMS+QMS lowers the barrier to entry for smaller firms by eliminating up-front software and implementation costs. Plus, with our solution, clients pay per user – so you’ll only pay for what you need.
In this way, the Pharma IT Cloud EDMS+QMS acts as a digitization accelerator for firms who may otherwise be unable to utilize electronic document and workflow systems. The system is flexible and can grow in parallel with Ascelia Pharma, allowing the company to scale up without a costly large-scale migration or re-validation.
Plus, while Pharma IT manages initial configuration and on boarding, Ascelia Pharma remains the sole owners of their intellectual property, eliminating the need for third-party data managers.
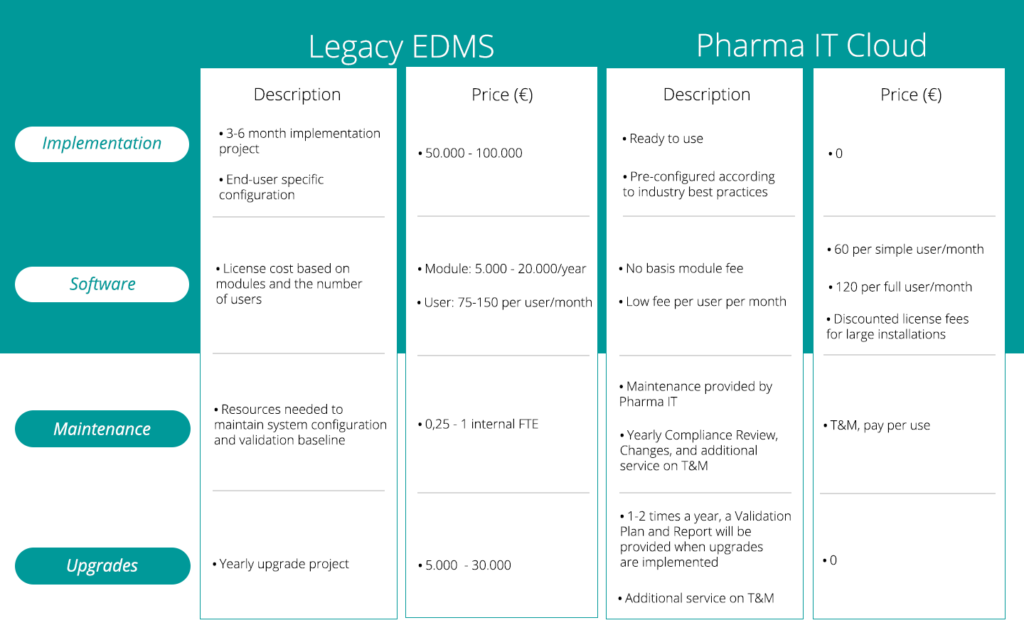
Pharma IT absorbs the role of IT System implementation, launch, and maintenance at a much lower cost. Plus mitigate the risk of non-compliance by leveraging our industry expertise. Beyond cost-efficiency, rapid system deployment and training means our clients benefit from a faster return on investment.
Taken together, the cost-effectiveness, flexibility, security, support, speed, and risk mitigation provided by the Pharma IT Cloud solution proved to be the perfect match for Ascelia Pharma.
Ascelia Pharma is now live on the Pharma IT Cloud and continues to benefit from the operational support and maintenance we provide. Our solution is easy to use, scalable, and tailored specifically to the demands of meet the pharma and biotech industries.
If your team is facing challenges similar to Ascelia Pharma, or is looking for an alternative to legacy EDMS+QMS solutions, do not hesitate to reach out to our team.
Jakob Juul Rasmussen is the Co-Founder and Managing Director of Pharma IT. He has 20 years of experience managing IT projects within the Pharma, Finance, and Public sectors.